



NucleoCounter® NC-200
The NucleoCounter® NC-200™ is a high precision automated cell counter utilizing state-of-the-art image cytometry. It counts suspension cells, aggregated cells, CAR-T cells, stem cells and cells on microcarriers.
Key features of NucleoCounter NC-200
- Precise counting of:
- Aggregated cells
- Cells growing on microcarriers
- Adipose-derived stem cells
- T cells (CAR T) - GMP validated and 21 CFR part 11 ready
- No service agreement, no calibration, no maintenance
- Eliminates human errors with Via1-Cassette™
- Unlimited software licenses
With user-adaptable protocols and specialized assays, the NucleoCounter® NC-200™ provides an all-in-one solution for cell counting and cell viability determination.
The Via1-Cassette™ is used with this counter for automated staining and sample handling. The cassette is volume calibrated to ensure high precision and cell reproducibility.
Innovative Via1-Cassette™ - eliminates human error

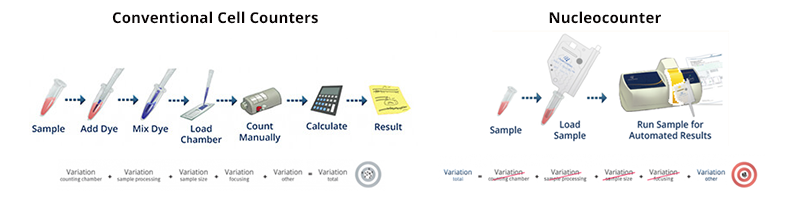
When counting manually the subjective evaluation of the definition of a cell introduces bias to the result. The NucleoCounter® NC-200™ is designed to limit human interference in counting. With the Via1-Cassette™, all errors introduced during pipetting and staining are avoided. The Via1-Cassette™ includes an in-built pipette and the immobilized fluorescent dyes acridine orange and DAPI automatically stain the total and dead cell populations, respectively. The Via1-Cassette™ is volume calibrated ensuring a high precision in determining cell count and viability. In total, this patented one-step viability and cell count takes less than 50 seconds.
Combining simplicity and innovation cell counting
Using the automated cell counter NC-200™ is a simple 3-step procedure: Load the cassette with cell suspension, insert the cassette into the NucleoCounter® NC-200™ and press 'RUN' (Figure 1). The results are automatically stored with the accompanying NucleoView™ software and can be accessed anytime.
Visual verification of cell count
Even for simple cell count and viability assays it is essential to define the right cell population to count. The major advantage of image cytometry with the NC-200™ is the possibility to link between the images obtained and the fluorescence plots. With the intuitive accompanying NucleoView™ software it is easy to define the counting gates precisely and thereby only count the right events inside the right populations – for both total and dead cell populations.
The accompanying NucleoView™ software allows for easy coupling between the obtained image, plots showing fluorescence and settings of counting gates.
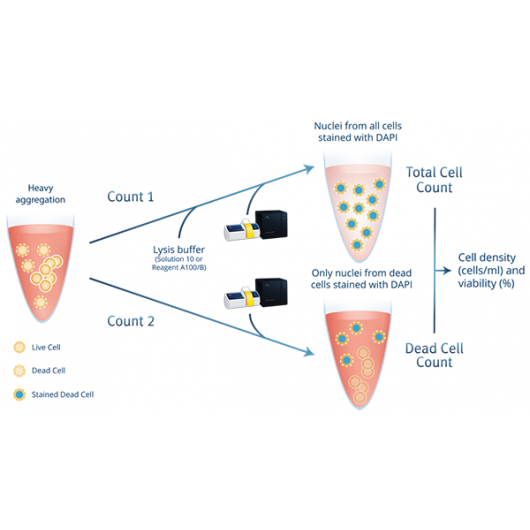
Precise cell counter for heavily aggregated cells
With the aggregated cells assay the NucleoCounter® NC-200™ counts nucleated cells by fluorescent staining of the DNA. Based on a carefully developed lysis buffer system, ChemoMetec A/S offers an optimized 2-step protocol for counting and determining viability of heavily agggregated cells. In the first step, all cells are lysed and counted based on a fluorescent DNA stain. In the second step, only dead cells are counted. Within just 2 minutes, the NucleoCounter® NC-200™ will report the precise viabilty and cell count.
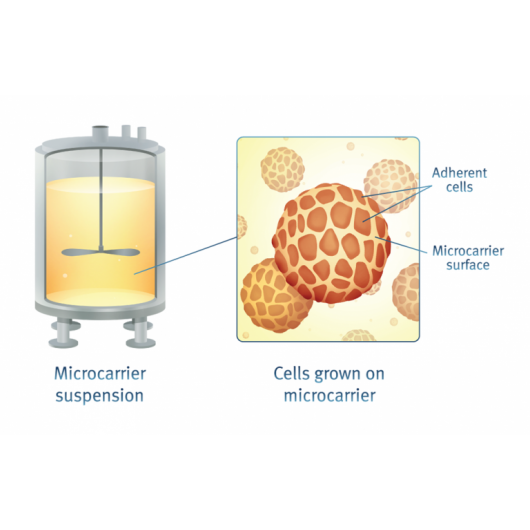
Optimized cell counting protocols for cells on microcarriers
The NucleoCounter® NC-200™ offers a unique protocol to count and determine viability of cells grown on microcarriers without the need of detachment. With the reagents A100 and B, cells are lysed bringing the nuclei into suspension to be counted using the NC-200™ (Figure 4.). Determination of the number of dead cells is performed based on the assumption that all dead cells are free in suspension and no longer attached to the microcarrier.
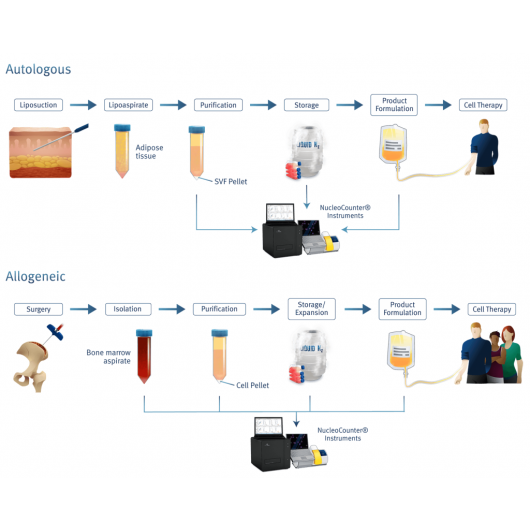
Precise cell counter for adipose-derived stem cells
The adipose-derived stem cells (ASCs) and the mesenchymal stem cells (MSCs) from the stromal vascular fraction (SVF) are of massive interest as these multipotent stem cells are easily accessible and can be purified in sufficient numbers directly from adipose tissue. The determination of cell count and viability of ASCs directly from SVF is especially challenging, as micelles and microvesicles easily can be mistaken for cells when counting manually or when counting with many automatic cell counters.
The NucleoCounter® NC-200™ counts only nucleated cells overcoming this challenge entirely (Figure 5). By addition of a lysis buffer, cells and other membrane enclosed particles will be lysed, leaving only the free nuclei in suspension for staining and counting with DAPI and the NucleoCounter® NC-200™.
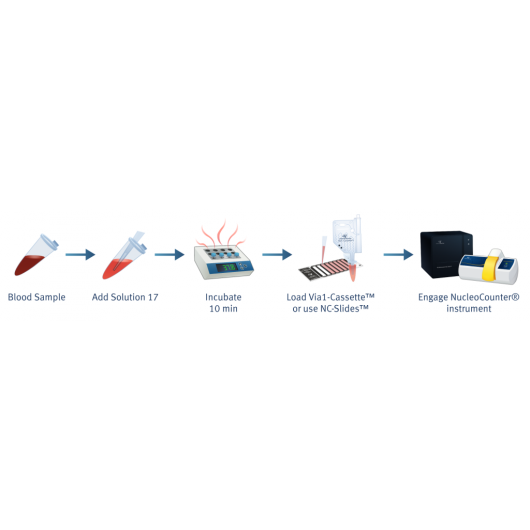
Counting of PBMC's (Peripheral Blood Mononucleated Cells) in whole blood samples
Peripheral Blood Mononucleated cells (PBMCs) are cells with one round nuclei. The PBMCs are a critical component of the immune system and are therefore of massive interest to versatile biological fields of research. Standard methods to separate and evaluate the content of PBMCs in a cell sample include Ficoll separation followed by counting and viability determination of the number of PBMCs in the sample. Instead, the determination of cell count and viability of PBMCs can be performed directly from the blood sample without the time consuming process of purification.
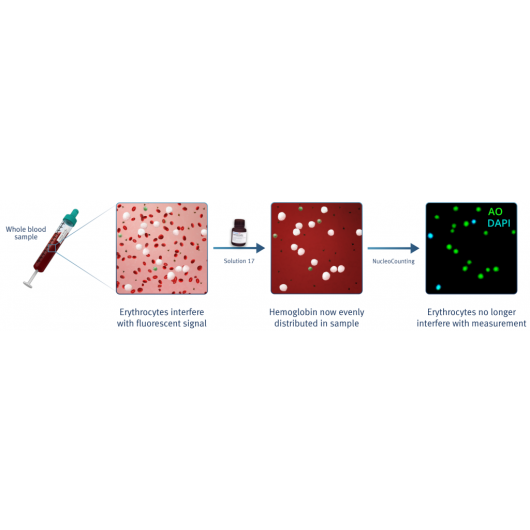
With the NucleoCounters® NC-200™, NC-250™ and NC-3000™, the total viability and cell count of leukocytes can easily be determined in cell samples containing both platelets and red blood cells (Figure 6).
By addition of blood lysis buffer Solution 17, red blood cells are lysed and hemoglobin diluted, leaving only the nucleated cells to be stained by acridine orange (green) and DAPI (blue). Platelets will not be counted as they will only be very weakly stained. Image cytometry with the NucleoCounter® family instruments NC-200™, NC-250™ or NC-3000™ allows for fast, precise and easy viability and cell count determination of leukocytes directly in peripheral blood samples.
GMP Validation and 21 CFR part 11-ready
The NucleoCounter® NC-200™ is designed to be easily implemented in GMP and 21 CFR part 11 compliant laboratories. The in-built protocols for IQ/OQ/PQ ensure control of consistent operation. Standardized pdf-reports, user control and audit trails allow for documentation of all events. Together with the high reproducibility and consistency in measurements, the NucleoCounter® NC-200™ is the ideal choice for automated cell counting in GMP and 21 CFR part 11-compliant laboratories.
| Loading volume | 60 μl is loaded into the Via1-Cassette™ |
| Measurement volume | 1,4 μl in the measurement chamber of the Via1-Cassette™ |
| Analysis time | 50 seconds (one step), 120 seconds (two step) |
| Measurement range | 5 x 103 to 1 x 107 cells/ml |
| Optimal range | 5 x 104 to 5 x 106 cells/ml |
| Size | 38 x 26 x 22 cm (W x H x D), weight 3 kg |
| Software | NucleoView™ NC-200 computer software for documentation and presentation |
- NucleoCounter® NC-200™
- Laptop Stand for NC-200™ and labtop/tablet
- License for 21 CFR Part 11
- Via1-Cassette™ - per box (100 pcs.)
2014
Krabbe C, Bak ST, Jensen P, et al.
PLoS One, 2014 May 2;9(5):e96465
Cell Type: Stem Cell Field: Neurobiology
Assay: Cell count & viability
Jackson P, Kling K, Jensen KA, et al.
Environmental and Molecular Mutagenesis, 2014 epub
Cell Type: Epithelia Cells Field: Toxicology
Assay: Cell count & viability
Lei Y, Jeong D, Xiao J, Schaffer DV.
Cellular and Molecular Bioengineering, 2014 Jun;7(2):172-183.
Cell Type: PS cells, Stem Cell Field: Stem Cell Research
Assay: Cell count & viability
Otsuji TG, Bin J, Yoshimura A, et al.
Stem Cell Reports, 2014 Apr 24;2(5):734-45
Cell Type: PS cells, Stem Cell Field: Stem Cell Research
Assay: Cell count & viability
2013
Lei Y, Schaffer DV.
Proceedings of the National Academy of Sciences (PNAS), 2013 Dec 24;110(52):E5039-48
Cell Type: Stem Cell Field: Stem Cell Research
Assay: Cell count & viability
2011
Enhanced immunomodulatory activity of gelatin-encapsulated Rubus coreanus Miquel nanoparticles
Seo YC, Choi WY, Lee CG, et al.
International Journal of Molecular Sciences, 2011;12(12):9031-56
Cell Type: Peripheral Blood Mononuclear Cell (PBMC) Field: Immunology
Assay: Cell count & viability